About Us
GAP EnviroMicrobial Services Ltd. (GAP) specializes in environmental microbiology laboratory services including testing, analysis, and research in areas of surface water, wastewater treatment, disinfection efficacy, and microbial detection. GAP was established in 1996, and has grown since that time in both size and in the variety of services we offer and has continuously provided quality analysis for industries, institutions, engineers, and consultants.
With a focus on quality, GAP maintains a state-of-the-art level 2 microbiology laboratory capable of analyzing a wide range of samples associated with environmental systems. Our analysis capabilities include classical microbiological methods with a focus on special projects including for disinfection efficay testing, UV reactor validation support using bacteriophage (MS2, T1UV, and T7) and Bacillus, Legionella monitoring, and third party witnessing.
We are ISO/IEC 17025 certified with accreditation through the Canadian Association of Analytical Laboratories (CALA) for specific parameters that include Bacteriophages in water for UV disinfection validation, Baceterial Filtration Efficiency (BFE), and culturable Legionella in water.
Quality Assurance
Quality System Policy Statement
We will continually improve the quality of our services through the implementation of a quality management system and ongoing training of our employees. Our objective on all projects is to meet and exceed the expectations of our clients by providing quality services in a responsive, safe, and cost-effective manner.
Quality Objectives
GAP EnviroMicrobial Services is ISO/IEC 17025 compliant with accreditation by the Canadian Association for Laboratory Accreditation (CALA), which ensure the laboratory carries out testing and equipment calibration activities that are in accordance with the ISO/IEC 17025 standard.
Proficiency testing is performed constantly by all members of the laboratory on all accredited parameters including Bacterial Filtration Efficiency, Legionella, and Bacteriophage, with results being reported back to CALA. Internal verification of analytical methods is also performed by comparison of results obtained between analysts and through duplicate analysis of samples.
All calibration is performed by contractors that use methods and equipment that are traceable to a national standards organization such as the National Institute for Science and Technology (NIST) and all calibrations are maintained per manufacturer recommendations. At GAP, we maintain current calibration on micropipettes, balances, timers, veneer callipers, steel rules, spectrophotometers, radiometers and probes, thermometers, laminar flow hoods and fume hoods. Annual internal calibration using NIST traceable instrumentation is also performed on all incubators, refrigerators, and freezers with continual temperature monitoring taking place using a computer controlled data logging system. Regular preventative maintenance is also performed on all incubators, refrigerators, and freezers to help reduce equipment down time that may interfere with sample analysis.
At GAP we use a pure water system for all media preparation that is constantly maintained and monitored by monthly analysis of key parameters. Before any media is used to analyze client (customer) samples it must undergo rigorous quality control to ensure proper performance.
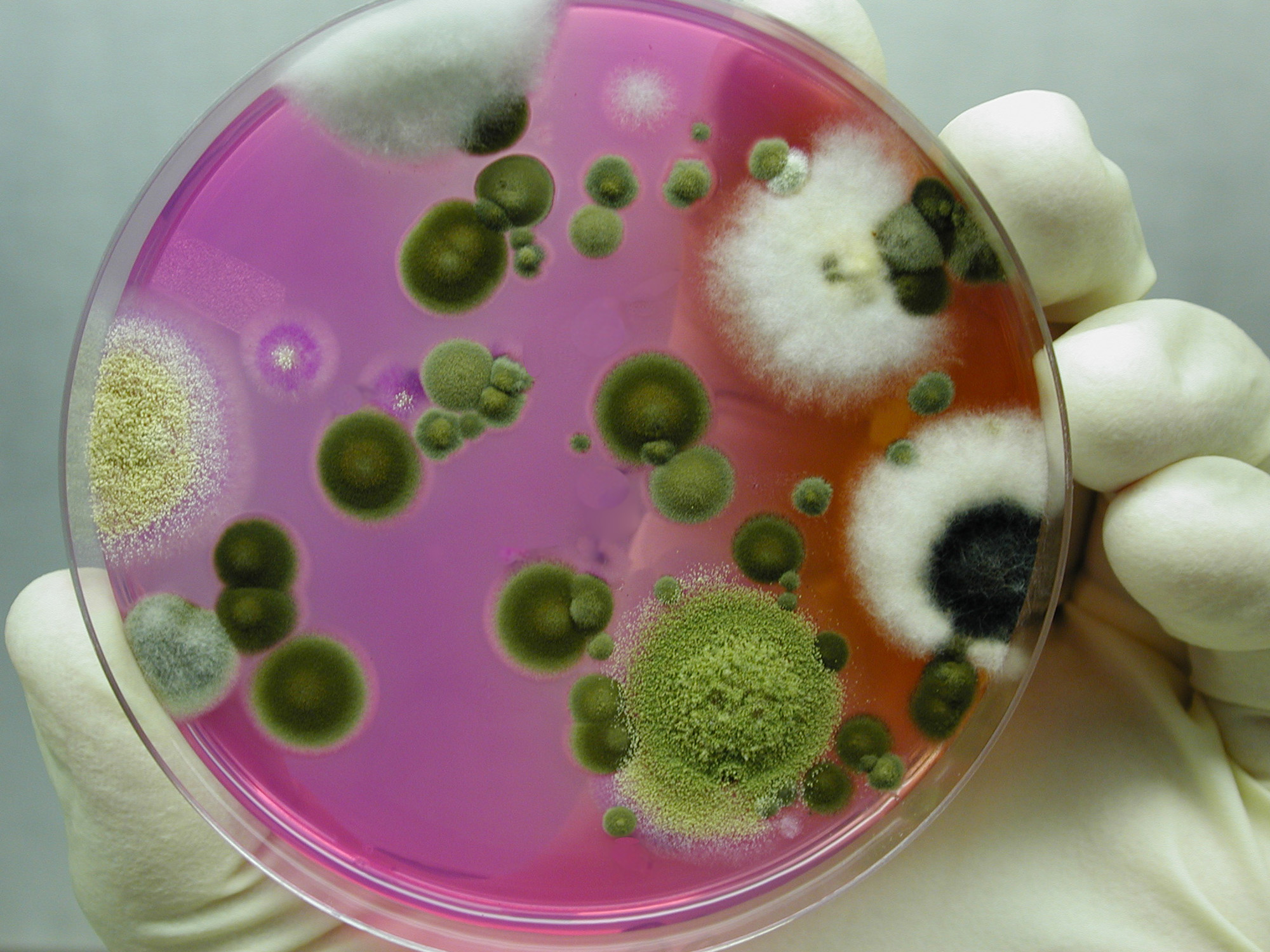
To help ensure environmental conditions do not affect analysis of samples, thorough cleaning and disinfection of the lab is a routine practice at GAP. This is ensured by monthly air quality monitoring throughout the lab for fungi and bacteria levels.
Accreditation Certificates
CALA Accreditation Certificate  [Adobe Acrobat PDF]
[Adobe Acrobat PDF]
ISO-ILAC-IAF Certificate  [Adobe Acrobat PDF]
[Adobe Acrobat PDF]
CALA Information
The Canadian Association for Laboratory Accreditation (CALA) is an accreditation board that provides international recognition, encourages quality standards of laboratories, and promotes understanding in clients and confidence of regulators.
CALA audits member laboratories to evaluate their performance in accordance with the ISO/IEC 17011 standard at regular intervals and grants accreditation based on the decision of the CALA Accreditation Council. This ensures laboratories are compliant with the ISO/IEC 17025 standard.
For a laboratory to maintain accreditation they must participate in inter-lab comparisons of accredited parameters either through CALA proficiency testing (PT) or through an alternate PT program. Any corrective actions that are undertaken must be done so in a specified time period and to the satisfaction of the CALA Advisory Panel.
CALA accreditation is recognized by almost 60 accrediting bodies in 40 countries around the world through a membership in the Asia Pacific Laboratory Accreditation Cooperation (APLAC) and a signatory to the APLAC Mutual Recognition Arrangement (MRA) and membership in the International Laboratory Accreditation Cooperation (ILAC) and a signatory to the ILAC MRA.
* Excerpts taken from www.cala.ca. Please see this website for additional information.
( back to top )